Abstract

INTRODUCTION: Adult T-cell leukemia/lymphoma (ATL) is an aggressive malignancy of regulatory T cells caused by human T-cell leukemia virus type 1 (HTLV-1) infection. Viral protein Tax is a key factor in malignant transformation. Peripheral blood mononuclear cells (PBMCs) collected from patients with smoldering and chronic-subtype ATL exhibited ex vivo spontaneous proliferation associated with Tax-dependent transactivation of two autocrine loops (IL-2/IL-2Rα, IL-15/1L-15Rα), and one paracrine loop (IL-9/IL-9Rα). Accordingly, antibodies directed against IL-2Rα, IL-9, and IL-15 inhibited proliferation of ATL cells. As the three cytokine/cytokine receptor loops act through downstream activation of the JAK1/3 and STAT5 signaling pathways, addition of the pan-JAK inhibitor tofacitinib and JAK1/2 inhibitor ruxolitinib also inhibited proliferation. Furthermore, ruxolitinib, when administered by continuous intravenous infusion, inhibited ATL cell proliferation in NOG mice.
METHODS: These observations led to a phase 2 open-label trial evaluating the safety and efficacy of ruxolitinib in patients with smoldering, chronic, or clinically indolent acute subtype ATL. Ruxolitinib was given at the FDA-approved dose of 20 mg orally twice daily for 28 days. Patients who responded to initial treatment could restart at the time of progression and be treated until progression. Primary objective was the clinical response rate per Revised Cheson Criteria and the International Consensus Meeting Criteria for ATL. Secondary objectives were 1) safety profile, 2) time to progression, 3) overall survival, and 4) duration of response off treatment.
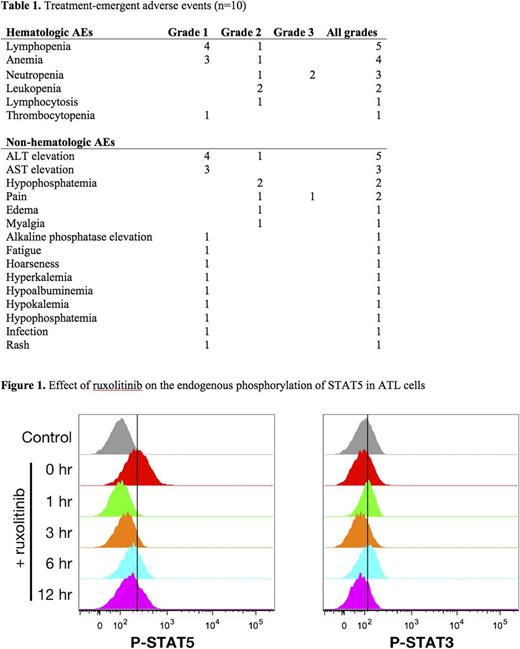
RESULTS: 5 patients with smoldering, 3 with chronic, and 2 with previously treated acute ATL were enrolled between October 2012 and July 2016. There were no grade 3/4 toxicities attributable to the study drug. Cytopenias were the most commonly reported adverse events, including two grade 3 neutropenias (table 1). One patient had a partial response lasting 2.4 months. Median time to progression was 1.6 months and median overall survival was 22.3 months. Three patients with partial or near-partial response were re-treated after a median of 5.7 months. To determine the time course of inhibition of the JAK-STAT pathway the concentration of phosphorylated STAT3 or STAT5 in ex vivo PBMCs was determined after an oral dose of 20 mg of ruxolitinib. Levels of phosphorylated STAT5 returned to baseline after 3-6 hours following the oral dose (figure 1), indicating that the pathway remained active for up to 18 hours per day when the drug was given twice daily.
DISCUSSION: Both of ruxolitinib's FDA approvals-for polycythemia vera and myelofibrosis-were based on reduction in spleen size. Its inhibitory effect on JAK2-mediated thrombopoietin signaling lead to thrombocytopenia as the main dose-limiting toxicity and precluded aggressive dose titration. In our study, single-agent ruxolitinib at the FDA-approved dose was safe but not effective in patients with indolent subtypes of ATL, likely due to lack of persistent JAK1/STAT5 inhibition. Further studies should therefore focus on 1) combinations of ruxolitinib with agents showing synergy on high-throughput screens, such as the Bcl-XL inhibitor navitoclax, and 2) more potent inhibitors of JAK1/3, like baricitinib, which was not associated with significant anemia or thrombocytopenia in a phase 3 trial. Ultimately, the future of indolent ATL treatment may lie in a multidrug combination that includes a specific JAK1 or JAK3 inhibitor. Considering the pervasive activation of the gamma cytokine JAK/STAT pathway in all T-cell malignancies, with up to 86% of patients manifesting pSTAT in the nucleus, the future of all T-cell malignancies may involve a specific JAK1 or JAK3 inhibitor.
Miljkovic: Pfizer Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal